Just 30 years after the publication of Moby Dick, a group of Alaskan whalers attempted to tame their own ocean giant. Their target was a male bowhead whale, the second largest mammal on Earth. The species were already famed for their amazing longevity: according to Inuit folklore, they could live “two human lifetimes”, and they were known to escape harpoons with their great strength.
The local Inuit claimed that the whales lived “two human lifetimes”
These whalers were armed with the latest technology, however – a “bomb lance”, fired with gunpowder on impact to pierce through the thick whale blubber. Yet it was not enough to conquer the whale. Three fragments of the lance caught in its side, but – like Moby Dick – it pulled away and escaped with little more than a surface wound.
The whale would continue to roam free for another 120 years, until 2007, when a group of Inupiat hunters finally caught the beast. They even found fragments of the original lance still embedded in the whale’s blubber.

Inuit hunters have long known that bowhead whales outlive almost every other creature (Credit: Getty Images)
According to many estimates, these whales live at least 150 years, and perhaps as long as 210. Apart from slightly leathery skin, a bit of excess blubber, and its battle scars, they show remarkably few ill-effects of long life, however. And that has made them of keen interest to doctors studying ageing.
“They live a lot longer than human beings, yet they are living in the wild, without going to the doctor or any of the perks of human society,” says Joao Pedro de Magalhaes at the University of Liverpool. “So they must be naturally protected from age-related diseases.”
If we could retard ageing even a little, it would have unprecedented human benefit
By studying these whales and other extraordinarily long-lived creatures, de Magalhaes and colleagues hope we can find new medicines that will similarly slow down the human body’s decay and delay death. “Ageing is a mystery – we know relatively little about it compared other biological processes, and yet it’s directly the greatest cause of suffering and death in the modern world,” says de Magalhaes. “If we could retard it even a little, it would have unprecedented human benefit.”
Vadim Gladyshev at Harvard University agrees. “This is the most important biological question, because the majority of chronic human diseases are the consequences of ageing. The way biomedical science is organised, it has mostly focused on particular diseases, like cancer, Alzheimer’s, or diabetes,” he says. “But if you delay ageing you could delay the incidence of all these diseases at once.”

Ageing may arise from a gradual wear and tear to our genes and cells – but there could be ways to mop up the damage (Credit: Getty Images)
Various factors are thought to contribute to ageing. First there’s the environment: no one can live in a pristine bubble, and so our DNA and other molecular machinery can become slowly damaged by harmful chemicals or radiation. Although our cells have some DIY toolkits that help fix the damage, eventually the devastation could become too widespread, leading to mutations that cause cells to turn into tumours.
We could also develop problems with our metabolism: like a furnace that needs regular cleaning, our cells produce a host of waste products as they burn energy. As the waste collects over time, it might disrupt crucial biological processes. “The amount of damage accumulates and the organism isn’t able to cope with it,” says Gladyshev.
You might think that ageing is an unstoppable force – but there are brakes to slow its progress
Finally, the body encounters problems regenerating organs: each chromosome has a bundle of DNA at its ends, called telomeres, which act a bit like the plastic tips of a shoelace, to stop it from fraying. The telomeres become shorter each time the cell divides, however, and eventually it could become so short that the cell begins to malfunction, or die. With this wear and tear, we are more vulnerable to various diseases.
Methuselah’s menagerie
All of which seems like an unstoppable force pushing us towards death – but several lines of evidence suggest there are brakes that can slow its progress. For instance, a common diabetes drug, metformin, can modestly slow ageing in mice. And simply changing one gene involved in cell metabolism in a roundworm can lead it to live many times longer than its parents; while it is unlikely the same changes would help more complex organisms, it hints that ageing is not beyond our control. “Ageing is a surprisingly plastic process that can be manipulated,” de Magalhaes says.
Natural selection has somehow pushed certain creatures to evolve their own elixir of life
Scientists like de Magalhaes and Gladyshev are now on the hunt for other candidates, using real-life Methuselahs as their guide. Across mammals alone, expected lifespan can vary 100-fold, from shrews that live for no longer than 1.5 years to the bowhead whales that can live for more than 200. It is as if, for various reasons, natural selection has somehow pushed certain creatures to evolve their own elixir of life.
“Metformin extends lifespan in mice modestly, but when you look at different species, the capacity of natural selection to extend lifespan is incredibly more powerful,” says de Magalhaes. “They will have probably evolved entirely different ways of living longer, and resisting cancer and other age-related diseases.” And each of those could lead to better medicine. Or as Gladyshev puts it: “Nature changes lifespan all the time, so the question is, how does it do it? And can we target those mechanisms, thereby increasing human longevity?”
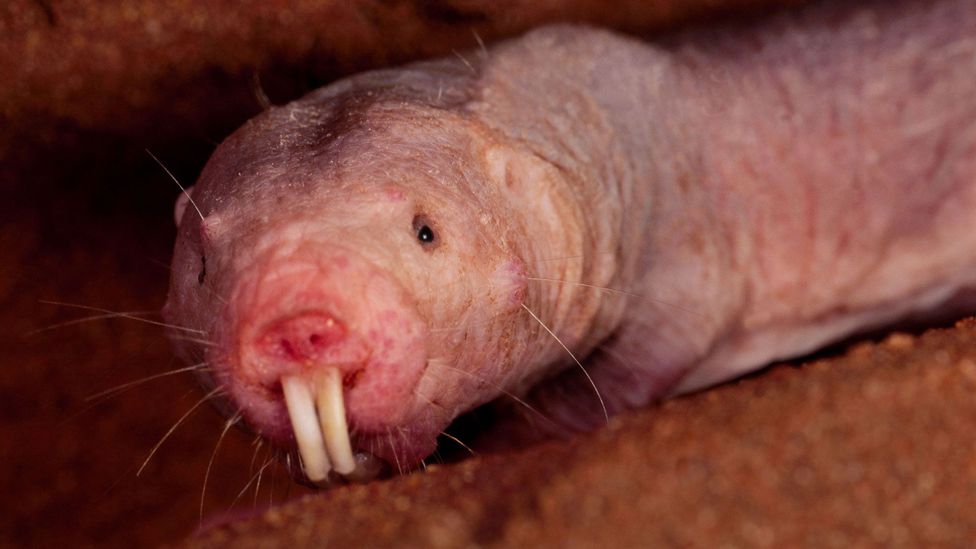
It’s not pretty, but the naked mole rat’s wrinkly and elastic skin may, ironically, hold the secret to its longevity (Credit: Alamy)
The Brandt’s bat lives more than 40 years, despite weighing little more than a sugar cube
The most interesting creatures are the extreme outliers; single species that seem to outlive even their closest relatives. They are not always as magnificent as the bowhead whale. With its wrinkled, hairless skin, the naked mole rat does not look like a poster child for successful ageing – yet it lives for up to 30 years, much longer than the two or three years that well-fed house mice can enjoy. In particular, the naked mole rat is unusually resistant to cancer – no tumours have yet been found in thousands of individuals studied in the lab. Even when they have been bathed in potent carcinogens, they remain cancer-free.
This might be partly explained by the fact that their cells tend to stop growing when they become too dense – a mechanism that stops tumours from replicating and growing out of control. The trait seems to arise from a particularly “heavy” version of a chemical known as hyaluronan. This molecule is part of the scaffolding surrounding cells, and might have originally evolved to make naked mole rat’s skin more elastic as they squeezed through their tight burrows. Today, it appears to be part of the signalling that stops cells from multiplying uncontrollably. In other words, even if a mutation would cause a tumour to form, the hyaluronan stops it from developing further.

Brandt’s bats live five times as long as other, closely related species (Credit: Alamy)
Gladyshev has also studied Brandt’s bat – a tiny creature that lives for more than 40 years, despite weighing little more than a sugar cube. “Considering the size, it’s the most extreme case,” says Gladyshev. He has found unusual mutations around its receptors for growth hormone and insulin-like growth factor – alterations that could offer further clues about ways to control metabolism in mammals, limiting the damage that normally comes with age.
Would the record-breaking bowhead whale reveal similar insights? The whale’s enormous size – 20 metres long and up to 100 tonnes in weight – creates some unique challenges that are of particular interest to biologists like de Magalhaes and Gladyshev. For instance, if its cells burnt energy at the same rate as mice cells, the excess heat would boil the surrounding water, so it has evolved to live with a slower metabolism and lower body temperature.
Such a huge body also puts you at enormous risk of cancer, thanks to simple mathematics: the more cells you have, the more likely you are to develop a harmful mutation. (Indeed, one study found that taller people are slightly more likely to develop cancer than shorter people, for this very reason.) And the problems become even greater the longer your life span. “When you live longer, you go through more divisions, so the likelihood of cancer increases hugely,” says Leonard Nunney at the University of California, Riverside, who researches the evolution of cancer.
Based on human rates of cancer, all large whales should all be riddled with tumours before they have even grown up – yet they continue to live for at least another century. This fact is known as “Peto’s Paradox”, and it suggests that the whales, like the naked mole rat, have some clever evolutionary tricks to deal with harmful mutations. “When put in the context of other animals, they really are outliers,” says de Magalhaes. “They must have tumour-suppressing mechanisms that we lack.”

Taller people are slightly more likely to develop cancer than shorter people, due to the simple fact that they have more cells to develop mutations (Credit: Getty Images)
The task was akin to shredding hundreds of thousands of copies of Moby Dick and then pasting the individual sentences back together
It was these mechanisms, and more, that de Magalhaes hoped to answer by peering into its genome. Initially, he struggled to find a way to sample the tissue. Eventually, however, he found a team who had already built links with Inuit hunters. To preserve their traditional way of life, indigenous societies around the Arctic are allowed to capture and kill a limited number of bowheads each year. Although they were initially suspicious of outsiders, the hunters eventually agreed for the scientists to sample some of the tissue collected from their kills.
Even once they had collected the material, the team still faced the extraordinary task of building the genetic sequence. Given the enormous volume of data, the task was akin to shredding hundreds of thousands of copies of Moby Dick and then pasting the individual sentences back together into a meaningful order.
The result is a series of leads that might just pave the way for future medicine. Of particular interest, they saw notable changes on a gene called ERCC1. This gene is known to code for a molecular toolkit that can patch up small areas of damage to the genome. It seems likely that the bowhead’s mutation makes it even more efficient at its job, perhaps preventing the build-up of harmful mutations that cause cancer.

Given their size, bowhead whales should all develop cancer before they reach adulthood – yet they can live for two centuries (Credit: Alamy)
The team also found changes to a gene called PCNA, which is involved in cell proliferation. It codes for a protein that acts as a kind of clamp, holding together the molecular machines that cause DNA to replicate. Bowheads have duplicated regions of the gene, and their mutations seem to help it interact with other parts of the toolkit involved in DNA repair. The team hypothesise that this single change could promote healthier cell growth without the damage that comes with age. Taken with other notable adaptations that might have helped the bowhead mop up cellular stress, these insights offer a first glimpse at the whale’s secrets to longevity.
‘Longevity signature’
Across the Atlantic at Harvard, Gladyshev recently performed his own study of the bowhead whales’ “transcriptome”; not just studying the genes, but also exploring how active they are. If you can see that certain genes are particularly active, then you know that it too might be playing an important role in ageing. Tellingly, he has found the same kinds of changes to insulin signalling that he also saw in Brandt’s bat. “It may change the metabolic setup of the cells, so that somehow it is longer-lived,” he says, “though the exact intricacies are still a matter of debate.” Together, the results provide a “longevity signature”, he says, that could then be used to guide future research.
The findings are attracting attention from some of the most important figures in medicine. Francis Collins, the director of the US National Institutes of Health has been impressed, writing that Gladyshev’s work brings us “right up to the threshold of new insights into healthier, longer life”.
The findings are attracting attention from some of the most important figures in medicine
Once over that threshold, there are many potential ways that these findings might improve treatments. Gladyshev suggests that we could see whether diet or exercise programmes could help to shift the body to match the longevity signature of the whale. For example, some have suggested that fasting, or “caloric restriction” slows ageing processes, and it might be interesting to compare whether it brings the kinds of metabolic changes that help the whale survive for so long. In this way, the bowhead whale could offer immediate guidance for the best ways to life longer.
The ultimate goal is to program the body so that cancers cannot form in the first place (Credit: Science Photo Library)
Alternatively, these long-lived creatures could inspire some more radical treatments. The first step, says de Magalhaes, will be to grow human tissue with some of the mutations seen in the bowhead whale, Brandt’s bat and naked mole rat. “If we change human proteins to resemble it, we can see if it improves DNA repair,” he says. “And I’d like to take bowhead whale genes and put them in mice to see if they live longer.”
Gene therapy could allow us to benefit from the helpful mutations that took millions of years to evolve
After these initial tests, the next hurdle will be to find a way to create the same changes in the enormously complex human body, perhaps with drugs that mimic the effects of the genes. In some cases, you could genetically engineer organisms like yeast to churn out the relevant proteins in large vats that could then be purified for human use, or to find drugs that mimic the effects. In the future, gene therapy could even allow us to tweak the DNA in living people; in an instant, we could benefit from the helpful mutations that took millions of years for the bowhead whale to evolve. Given the recent advances in gene therapy, he says “there’s no reason to think it’s not a possibility further down the line.”
Clearly, there will be trials ahead. Although we are relatively closely related in evolutionary terms, what works in a whale, or naked mole rat, may have limited or no benefit in the human body. “You can always find the different ways different organisms will suppress cancer – but whether they will be therapeutically useful, you just can’t predict in advance,” says Nunney. Nature’s answers to cancer evolved in a “serendipitous process” he says, with haphazard solutions arising from each organism’s unique circumstances. Even so, he welcomes this new approach to looking to nature for answers to medical issues. “I think cancer biologists are beginning to recognise that incorporating evolutionary ideas can be a fruitful endeavour.”
De Magalhaes and Gladyshev are under no illusions about the arduous journey – but they remain hopeful. “History is full of claims by experts that certain advances are impossible, only to be proven wrong soon after,” says de Magalhaes. Consider the state of medicine just 120 years ago, when some of today’s bowhead whales were still in their infancy. Back then life-threatening infections were just a fact of life. Today, antibiotics are taken for granted as the most basic healthcare.
Ultimately, de Magalhaes sees ageing as the ultimate disease – an illness that can be “cured” in its own right. “We’re not just extending the period of decrepitude,” he says. “We want 70 year olds with the health of a 50 year old – that’s the ultimate goal.” Maybe, in 2120, we will look back with wonder at his first steps to reach that goal.
David Robson is BBC Future’s feature writer. He is @d_a_robson on Twitter.














